胆道支架移位导致乙状结肠穿孔和盆腔脓肿
胆道支架移位导致乙状结肠穿孔和盆腔脓肿
内镜胆道支架置入术是用于治疗良恶性梗阻性黄疸公认的治疗方法。 通常对于胰腺癌导致的梗阻性黄疸患者放置塑料支架作为一项姑息治疗手段。 尽管在大多数情况下支架可通过肠道排出或在肠道中保留一段时间,但支架移位仍然被认为是并发症。 在极少数情况下,支架可能会导致乙状结肠穿孔和盆腔脓肿形成,特别是在伴有乙状结肠憩室或既往行腹部手术引起的腹腔粘连的患者中。本报道中此患者因胰头癌压迫胆管而行胆道支架,术后支架移位导致乙状结肠穿孔和盆腔脓肿的患者。
一名男子因休克,血压降至60/40mmHg就诊于A&E医院。患者浑身湿冷,多汗,体温为36.2°C。腹部出现全身性腹膜炎体征。全腹肌紧张和反跳痛,在左髂窝中反跳痛为著。静脉输液复苏后,患者排出100毫升深色尿液。在入院前14个月,他被诊断出胰头癌晚期伴肝转移,并且在内镜下行胆道支架置入术,并接受了姑息性化疗。10个月后CT扫描中证实患者支架移位,他需要在置入第二个支架。然而,入院前4周,即在支架置换后6个月进行的随访中,内镜逆行胰胆管造影术未能发现胆总管中的支架。此时没有进行进一步的检查。3天前,患者诉腹痛和便秘,并由他的肿瘤科医生给予泻药治疗。肿瘤医生团队没有对患者进行进一步的检查。(此段中时间点翻译尚有争议,大家可参考原文)。
入院时,患者的白细胞计数为15.6×10
3 / mL,C反应蛋白水平为99 mg / L,肝功能检查结果异常。立位平片没有穿孔证据,但腹部CT显示乙状结肠穿孔伴有盆腔脓肿(图1)。手术切除穿孔的乙状结肠,在引流脓腔及用温盐水彻底冲洗腹腔后行Hartman手术(图2)。术中,存在炎性渗出性腹腔积液(在骨盆内和乙状结肠周围化脓,并伴有盆腔脓肿和小肠与乙状结肠之间的粘连)。支架在乙状结肠憩室侧面穿出。由于广泛的盆腔炎症粘连,未进行腹腔镜检查,并且由于污染,不能进行一期修复。患者在重症治疗室中治疗5天后,由于多器官衰竭而恶化并死亡。
学习要点
内镜下胆道支架置入术是胆道系统短期和长期减压的良好措施。支架移位是一种罕见但严重的并发症,因为它可能导致危及生命的风险,特别是在伴有肠道病变的患者中,例如疝,憩室或腹部粘连,这些患者肠穿孔的风险增加。 R Diller建议不管是否有症状,对于伴有腹痛或败血症的有胆管支架置入史患者,支架移位是一项重要的鉴别诊断。如果怀疑支架移位,应常规进行腹部平片检查,并作为后续的检查手段。应遵循欧洲胃肠病学会(ESGE)关于胆道支架适应征和选择的指南,塑料支架不得作为胆道梗阻的长期处理方法。
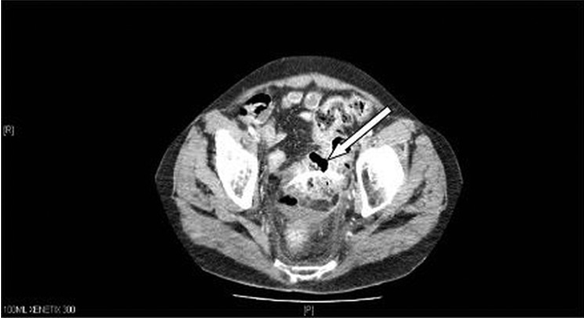
CT示支架穿透乙状结肠

乙状结肠切除标本显示胆道支架穿出肠外
Migrated biliary stent causing perforation of sigmoid colon and pelvic abscess.
Mady R F, Niaz O S, Assal M M. Bmj Case Rep, 2015(apr13 1).
Abstract
Endoscopically placed biliary stents are a well-established procedure for the treatment of benign and malignant causes of obstructive jaundice. A plastic stent is usually inserted in patients with obstructive jaundice due to pancreatic cancer as a short-term procedure. Stent migration has been reported as a complication, although in most cases the stent will pass through or remain in the bowel lumen for a period of time. In rare cases, the stent may cause sigmoid perforation and pelvic abscess formation, especially in patients with sigmoid diverticulae or abdominal adhesions due to previous surgery. We present a patient with sigmoid perforation and pelvic abscess due to distal migration of a biliary stent placed to decompress a pancreatic head carcinoma.
A man presented to the A&E department in shock, with blood pressure of 60/40; he was cold, clammy and sweating, and had a temperature of 36.2°C. His abdomen showed signs of generalised peritonitis. He exhibited guarding and tenderness all over his abdomen and was most tender in the left iliac fossa. After resuscitation with IV fluids, he produced 100 mL of dark urine. Fourteen months prior to this presentation, he had been diagnosed with advanced carcinoma of the head of pancreas with liver metastases and, following endoscopic insertion of a plastic biliary stent as short-term management, had received palliative chemotherapy. He required insertion of a second stent 10 months later due to stent migration, demonstrated on CT scan. A follow-up endoscopic retrograde cholangiopancreatography 6 months following stent replacement, 4 weeks prior to this presentation, however, failed to demonstrate a stent in the common bile duct. No further investigations were undertaken at that point. Three days before his presentation to A&E, the patient reported abdominal pain and constipation, and was given laxative treatment by his oncologist. No further investigations were performed on the patient by the oncology team.
On admission, the patient’s white cell count was 15.6×103/mL, his C reactive protein level was 99 mg/L and his liver function tests were deranged. There was no evidence of perforation on the erect chest radiograph, but CT of the abdomen demonstrated perforation of the sigmoid colon with an associated pelvic abscess (figure 1). The patient was treated by surgical resection of the perforated sigmoid and Hartman’s procedure was performed after drainage of the abscess cavity and extensive washing out of the peritoneal cavity with warm saline (figure 2). Intraoperatively, there was some reactive peritoneal fluid (suppurative in the pelvis and around the sigmoid, with pelvic abscess and adhesions between small bowel and sigmoid). The stent perforated the sigmoid through a diverticulum in the lateral aspect. A laparoscopy was not performed because of extensive pelvic inflammatory adhesions and primary repair was not an option because of the contamination. The patient spent 5 days in intensive treatment unit and then deteriorated and died due to multiorgan failure.
Learning points
Endoscopic insertion of biliary stents is a good procedure for short-term and long-term decompression of the biliary system. Stent migration is a rare but important complication, as it may result in life-threatening conditions, particularly in patients with comorbid intestinal pathologies such as herniae, diverticular disease or abdominal adhesions, who are at increased risk of intestinal perforation. R Diller recommended that a migrated biliary stent should be removed immediately irrespective of whether it is symptomatic.
Stent migration is an important differential diagnosis in any patient with a biliary stent presenting with abdominal pain or sepsis. Plain abdominal radiographs should be routinely performed if stent migration is suspected and as a follow-up tool.
The European Society of Gastrointestinal Endoscopy (ESGE) guidelines for the indications and choice of biliary stent should also be followed. Plastic stents must not be used as long-term management for biliary obstruction.
翻译:于廷廷 审校:张立超、侯森林